In the context of the coronavirus pandemic, the largest pharmaceutical companies from around the world are offering various antiviral drugs to prevent, reduce symptoms of the disease and reduce the risk of complications.
We offer you a list of drugs that are positioned as a drug for Covid-2019.
Please note that the effectiveness of these drugs against the new coronavirus has not yet been fully understood. And they all have side effects. When there is symptoms characteristic of the Wuhan coronavirus, you should immediately consult a doctor and discuss with him the advisability of taking one or another medication from this collection.
Drugs to fight coronavirus infection
10. Hydroxychloroquine
 Several antimalarial drugs are opening the top 10 drugs to combat coronavirus - hydroxychloroquine, chloroquine, mefloquine. It is not yet entirely clear how exactly the drugs fight the virus, but, as stated in the recommendations of the Ministry of Health on combating coronavirus, hydroxychloroquine somehow prevents harmful viruses from entering the cell and settling comfortably there for reproduction. For greater effectiveness, it can be prescribed in conjunction with the antibiotic azithromycin.
Several antimalarial drugs are opening the top 10 drugs to combat coronavirus - hydroxychloroquine, chloroquine, mefloquine. It is not yet entirely clear how exactly the drugs fight the virus, but, as stated in the recommendations of the Ministry of Health on combating coronavirus, hydroxychloroquine somehow prevents harmful viruses from entering the cell and settling comfortably there for reproduction. For greater effectiveness, it can be prescribed in conjunction with the antibiotic azithromycin.
However, antimalarial drugs have one big drawback - these drugs are harmful to the heart. Therefore, they should be taken only under the supervision of a doctor and regularly monitor the indicators of the cardiovascular system, for example, by taking ECG readings at least once every 5 days.
9. Paracetamol - WHO recommendation
 By itself, paracetamol against COVID-19 is useless, but it perfectly helps to fight the symptoms of the disease - temperature and inflammatory processes in the body. The World Health Organization has officially recommended that people who experience the first symptoms of coronavirus infection take paracetamol.
By itself, paracetamol against COVID-19 is useless, but it perfectly helps to fight the symptoms of the disease - temperature and inflammatory processes in the body. The World Health Organization has officially recommended that people who experience the first symptoms of coronavirus infection take paracetamol.
This does not mean that paracetamol itself is that good. WHO proposed it as an alternative to ibuprofen. According to some studies, the use of ibuprofen for acute respiratory infections can cause unwanted side effects and ultimately make patients only worse.
8. Remdesivir
 A new American drug, the results of which against COVID-19 look very promising, at least from the point of view of the WHO and the US Department of Health. The latter department even bought it many months in advance from the Californian company Gilead Sciences.
A new American drug, the results of which against COVID-19 look very promising, at least from the point of view of the WHO and the US Department of Health. The latter department even bought it many months in advance from the Californian company Gilead Sciences.
Perhaps the drug will soon appear in Russia as well - at the end of September Gilead Sciences and Pharmstandard signed an agreement on a possible partnership and supply of Vekluri (trade name of remdesivir) to our country.
The drug was tested in China, where patients who took it recovered, on average, one third faster than normal coronavirus patients. True, remdesivir turned out to be the most effective in treating mild to moderate coronavirus disease, but mortality remained at the same level.
7. Vaccines mRNA-1273 and BNT162
 In total, several messenger RNA-based coronavirus vaccines are currently being developed in the world. Compared to traditional vaccines, which are usually antigen-based, mRNA vaccines can be produced much faster.
In total, several messenger RNA-based coronavirus vaccines are currently being developed in the world. Compared to traditional vaccines, which are usually antigen-based, mRNA vaccines can be produced much faster.
They work like this: this very mRNA enters the body, which, if greatly simplified, builds a factory for the production of antigens in the human body and stimulates immunity against coronavirus. That is, the vaccine itself will not cure the disease, but it can prevent it.
At the moment, BNT162 vaccines from the German companies BioNTech and Pfizer and mRNA-1273 from the American Moderna have entered the home stretch. Based on clinical trials, vaccines have proven to be effective enough to prevent disease.
However, mRNA vaccines must be stored at very low temperatures, no higher than -70 °. Conventional pharmacies do not have such capabilities, which leaves the use of mRNA-1273 and BNT162 for medical centers - hospitals and laboratories.
6. Polyclonal anti-SARS-CoV-2-hyperimmune globulin (H-IG)
 The author of the development is the Japanese pharmaceutical company Takeda Pharmaceutical. They decided to take a non-standard path and use blood plasma as a cure for coronavirus. More precisely, hyperimmune globulins, which are obtained from the blood plasma of people who have recovered from the coronavirus.
The author of the development is the Japanese pharmaceutical company Takeda Pharmaceutical. They decided to take a non-standard path and use blood plasma as a cure for coronavirus. More precisely, hyperimmune globulins, which are obtained from the blood plasma of people who have recovered from the coronavirus.
The drug began to undergo clinical trials only in September this year, and they were sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) in the United States. We have already recruited 500 "guinea pigs" from different countries of the world - from Denmark to Argentina. Plasma results will be compared with remdesivir and placebo.
5. Recombinant interferon alpha - recommendation of the Ministry of Health of the Russian Federation
 Interferons are special molecules that human bodies produce in response to viruses entering the body. They can be used by synthesizing artificial ones on their basis, which are then taken by sick people. As they say, they can both facilitate the passage of the disease, and even serve as a kind of vaccine, preventing the very development of the disease.
Interferons are special molecules that human bodies produce in response to viruses entering the body. They can be used by synthesizing artificial ones on their basis, which are then taken by sick people. As they say, they can both facilitate the passage of the disease, and even serve as a kind of vaccine, preventing the very development of the disease.
Among such drugs, the Ministry of Health considered the use of injections of interferon alfa 2b (IFN-a2b) effective. The drug has immunomodulatory, anti-inflammatory and antiviral effects.
4. Avigan (Favipiravir)
 The drug was created by the Japanese pharmaceutical company Fujifilm Holdings specifically to fight the flu. The drug had many side effects (for example, it caused disorders in the development of the fetus), so it was not reported to the pharmacy chain. However, the Japanese government preferred to keep some medicine in its bins in case a flu epidemic hit the country.
The drug was created by the Japanese pharmaceutical company Fujifilm Holdings specifically to fight the flu. The drug had many side effects (for example, it caused disorders in the development of the fetus), so it was not reported to the pharmacy chain. However, the Japanese government preferred to keep some medicine in its bins in case a flu epidemic hit the country.
An epidemic has come to the Land of the Rising Sun, but not the flu. Japanese doctors began clinical trials of the drug, which ended just a couple of days ago, and right now they are analyzing the results. However, the Chinese, in whose hands the Japanese medicine fell, confirm that it is quite effective - however, against not the virus itself, but the pneumonia caused by it.
3. Coronavir
 Russia also has its own analogue of the drug with the main active ingredient favipiravir. It is produced by the same R-Pharm company that previously produced large quantities of lopinavir and ritonavir. The only pity is that this combination turned out to be ineffective against Covid-2019.
Russia also has its own analogue of the drug with the main active ingredient favipiravir. It is produced by the same R-Pharm company that previously produced large quantities of lopinavir and ritonavir. The only pity is that this combination turned out to be ineffective against Covid-2019.
On September 17 this year, Coronavir was registered in the register of medicines of the Russian Federation.Based on the results of a clinical study, this coronavirus drug is showing downright amazing results:
- more than half of the patients experienced a sharp improvement,
- there were practically no side effects, with the exception of a slight increase in urine acidity.
Very soon, the medicine will appear in pharmacies at a “modest” price of 11,000 rubles per pack.
2. Areplivir
 Another clone (that is, a generic is a copy of the original drug, but produced by another company) of the Japanese influenza drug, which was already mentioned in the fourth point of the ranking - favipiravir. This time the drug is produced by the Promomed company. At the end of September, the drug arrived at the pharmacy at a price of 12 thousand rubles per pack and was immediately sold out.
Another clone (that is, a generic is a copy of the original drug, but produced by another company) of the Japanese influenza drug, which was already mentioned in the fourth point of the ranking - favipiravir. This time the drug is produced by the Promomed company. At the end of September, the drug arrived at the pharmacy at a price of 12 thousand rubles per pack and was immediately sold out.
However, employees of the Federal Antimonopoly Service have already turned their attention to this drug and sent requests to pharmaceutical companies asking them to explain why it is so expensive? To which the manufacturers said that the drug is not included in the list of vital and essential drugs, so the state cannot prevent them from setting any price for it. And it's expensive - because it is effective and “saves lives”!
True, evil tongues claim that favipiravir is actually a slightly modified pyrazinamide, a well-known drug in Russia, a cheap drug for the treatment of tuberculosis. It cost 180 rubles per pack in pharmacies. It is rumored that pyrazinamide has mysteriously disappeared from the shelves lately. It is likely that all of the raw materials were used for the latest drugs to combat COVID-19.
1. Avifavir
 The list of Russian versions of favipiravir is crowned with the very first swallow on the Russian market - Avifavir from ChemRar and the Russian Direct Investment Fund. Already in early May, the CEO of the RDIF announced that favipiravir could become a long-awaited panacea against coronavirus infection. And in June 2020, several tens of thousands of packages arrived at Russian hospitals, but the drug has not yet appeared in retail sales.
The list of Russian versions of favipiravir is crowned with the very first swallow on the Russian market - Avifavir from ChemRar and the Russian Direct Investment Fund. Already in early May, the CEO of the RDIF announced that favipiravir could become a long-awaited panacea against coronavirus infection. And in June 2020, several tens of thousands of packages arrived at Russian hospitals, but the drug has not yet appeared in retail sales.
The drug has shown efficacy, which is traditional for Russian anti-coronavirus drugs based on favipiravir. More than half of the patients were cured after five days, and after 10 days - 90%. True, the sample was only 60 people, but, as the RDIF representative argued, sometime earlier the drug had already undergone clinical trials, in which more than 1200 people participated.
The Ministry of Health of the Russian Federation reacted positively to the release of drugs based on favipiravir on the market, but many doctors look at them with skepticism. They are confused by the abundance of side effects in the Japanese version of the drug, which suddenly miraculously disappeared from Russian drugs.
At a minimum, pregnant women should not take these medications because of the teratogenic effects on the fetus, as well as people with renal and hepatic impairment. And people without chronic diseases - only under the supervision of a doctor. It is also recommended to take blood tests and undergo a medical examination after the end of the course of taking the drug.
Sputnik V coronavirus vaccine is released into civilian circulation: when to expect mass vaccination
 At the beginning of September 2020, vaccination against the coronavirus of the population in occupational risk groups began in Russia - these are doctors, teachers, etc.
At the beginning of September 2020, vaccination against the coronavirus of the population in occupational risk groups began in Russia - these are doctors, teachers, etc.
However, so far there are few batches of the drug, and it is expected that mass vaccination of Russians from risk groups will take place in October-November this year. As a priority, the vaccination will involve police officers, traffickers, social security workers, and other categories of citizens working in close contact with people.
Simultaneously with vaccination against COVID-19, the third phase of clinical trials of "Sputnik V" will begin. It will assess the epidemiological efficacy of the vaccine, and will be conducted according to the standards of randomized, double-blind trials.This means that neither the doctors nor the participants in the study (and there will be approximately 30-40 thousand people) will not know who received the real drug, and who the "dummy" - placebo.
After the post-registration study is completed, a massive vaccination campaign for the residents of Russia will begin. According to experts, this will be the end of January-beginning of February 2021.
At the same time, there is no talk of vaccination of children yet, for them the drug will be available after additional research. In addition, the Russian vaccine is not yet intended for people over 60, at least until the completion of the third stage of its study.
Sputnik V is a development by scientists from the Gamaleya Center. The vaccine is two-component, after the first injection, you need to wait three weeks and do the second. It has already been included in the list of drugs, the distribution of which is controlled by the state.
This is how the vaccine works:
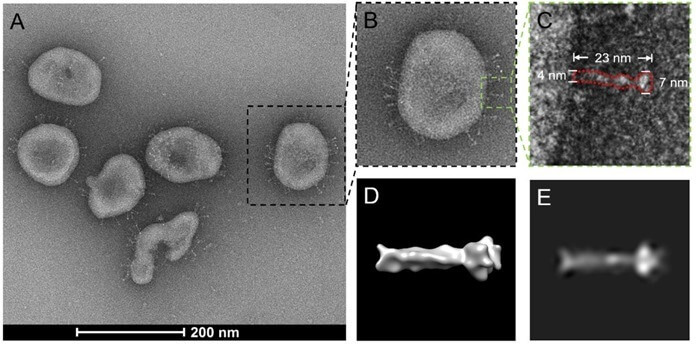
- Thanks to the introduction of Sputnik V, the production of the peak COVID-19 protein will begin in the cells of the vaccinated person.
- The peaks (B, C, D in the picture) are the objects that dot the surface of the coronavirus (in the picture it is indicated by the letter A). They help it attach to healthy cells.
- The vaccinated immune system recognizes the foreign protein and starts producing antibodies. This is how immunity to coronavirus is formed.
- It is impossible to get sick with COVID-19 after receiving a vaccine, since it contains only a fragment responsible for the creation of peak proteins, from which a full-fledged virus cannot collect.
Drugs that turned out to be useless against 2019-nCov
3. Ribavirin
 The top 3 ineffective drugs against Covid-19 open up a drug that the Russian Ministry of Health once recommended as a potential treatment for the new coronavirus. In an interview with RBC, the pulmonologist, academician of the Russian Academy of Sciences Alexander Chuchalin told about its uselessness for this purpose.
The top 3 ineffective drugs against Covid-19 open up a drug that the Russian Ministry of Health once recommended as a potential treatment for the new coronavirus. In an interview with RBC, the pulmonologist, academician of the Russian Academy of Sciences Alexander Chuchalin told about its uselessness for this purpose.
He said that after the studies, the WHO came to the conclusion that ribavirin was ineffective against coronavirus infection ("atypical pneumonia") that arose in 2002. In 2012, there was another outbreak of coronavirus, the biological source of which was camels. Again, ribavirin was not effective. And so far there is no evidence that ribavirin will be useful against the Wuhan coronavirus.
2. Lopinavir-ritonavir
 The combined antiviral drug, which is produced under different names (the most famous is Kaletra), has proven ineffective in cases of severe coronavirus infection. Such conclusions of the Chinese researchers were published on March 18 in the New England Journal of Medicine.
The combined antiviral drug, which is produced under different names (the most famous is Kaletra), has proven ineffective in cases of severe coronavirus infection. Such conclusions of the Chinese researchers were published on March 18 in the New England Journal of Medicine.
A study of 199 patients with severe complications caused by Covid-2019 did not show the effect of lopinavir-ritonavir on reducing mortality, the rate of improvement in the condition of the subjects or the length of their hospital stay.
1. Arbidol
 Since the demand for drugs that help with coronavirus is very high, OTCPharm decided to “be in trend” and announced its drug Arbidol effective in combating Covid-2019.
Since the demand for drugs that help with coronavirus is very high, OTCPharm decided to “be in trend” and announced its drug Arbidol effective in combating Covid-2019.
However, such a bold statement aroused the interest of not only consumers, but also the Federal Monopoly Service. Which found that OTCPharm violates the requirements of the law "On Advertising".
According to the FAS Commission, the advertising message about the action of "Arbidol" against the Wuhan coronavirus goes beyond the indications contained in the instructions for use. The penalty may be a fine of 200-500 thousand rubles.